Please see below the first edition of our STEM newsletter. We aim to publish a new edition each month about the various STEM workshops, activities, trips and masterclasses taking place at ACS, which we hope will inspire students and make science more approachable. Most articles in this newsletter were written by students.
Below you can read about some other STEM activities and workshops:
- An Imperial March
- Making a volcano
- Sublimation of Dry Ice
- Marshmallows and physics
- Bread is Made
- The Maker Challenge
- Centre of Mass and Torque
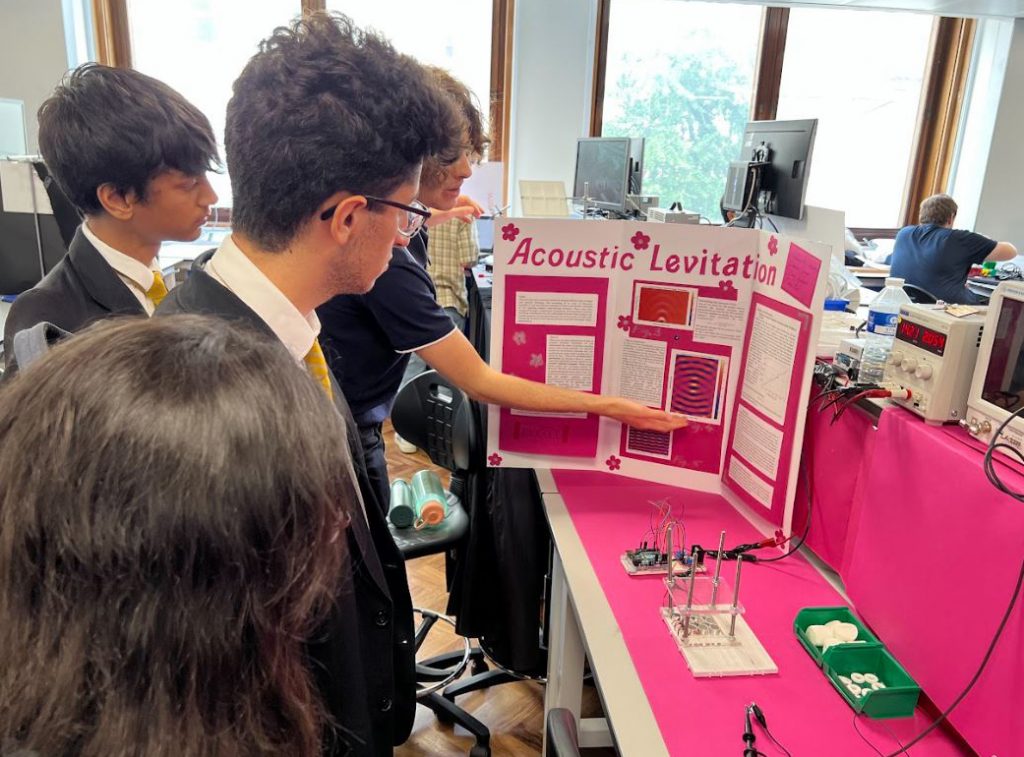
On Tuesday 20 June, thirty-five students from Year 11 and Year 12 attended the Physics Project Open Day hosted by the Imperial College London. Students attended two lectures by top university researchers. The first lecture was about the European Space Agency's 'Jupiter Icy Moons Explorer' mission (JUICE) and the second lecture about Solar Power. In between lectures, our students explored hundreds of Physics projects displayed by first-year undergraduate students developed in partnership with researchers. Please see below a detailed account of the visit written by one of our Year 11 students.
It was a temperate Tuesday morning when 35 Year 11 and Year 12 Alperton students left for Imperial College London to attend a Physics Project Open Day. Not even the crowded and sweltering Piccadilly Line could quash the excitement. After some time walking, we finally arrived at the prestigious Physics campus. The air itself felt light, knowledge racing through it like delocalised electrons.
 Imperial College London’s annual Physics Project Open Day aims to help aspiring young Physicists gain insight into the demands and expectations of reading Physics at a world-class institution. During the first year of studying at Imperial College, undergraduates partner with researchers to innovate and design a project within their chosen field such as electrical circuits or infrared astronomy. A showcase of these projects to fellow university students, staff and visitors, such as ACS students, makes for a thrilling event.
Imperial College London’s annual Physics Project Open Day aims to help aspiring young Physicists gain insight into the demands and expectations of reading Physics at a world-class institution. During the first year of studying at Imperial College, undergraduates partner with researchers to innovate and design a project within their chosen field such as electrical circuits or infrared astronomy. A showcase of these projects to fellow university students, staff and visitors, such as ACS students, makes for a thrilling event.
The day at Imperial began with a lecture on the potential existence of extraterrestrial life. We were told about the Jupiter Icy Moons Explorer (JUICE Project) and how a spacecraft launched on the 14th April 2023 studies three of Jupiter’s huge icy Galilean moons: Europa, Ganymede and Callisto. The project manager Vincent Poinsignon, described how “The goal is to investigate whether there are liquid oceans under these icy crusts which might harbour organic components or even life.” We learnt how this extraordinary project consisted of 11 scientific experiments, with a vital instrument having been built at Imperial College itself. It is astounding to think that this university, merely 7.4 miles away from school, is where life changing things happen.
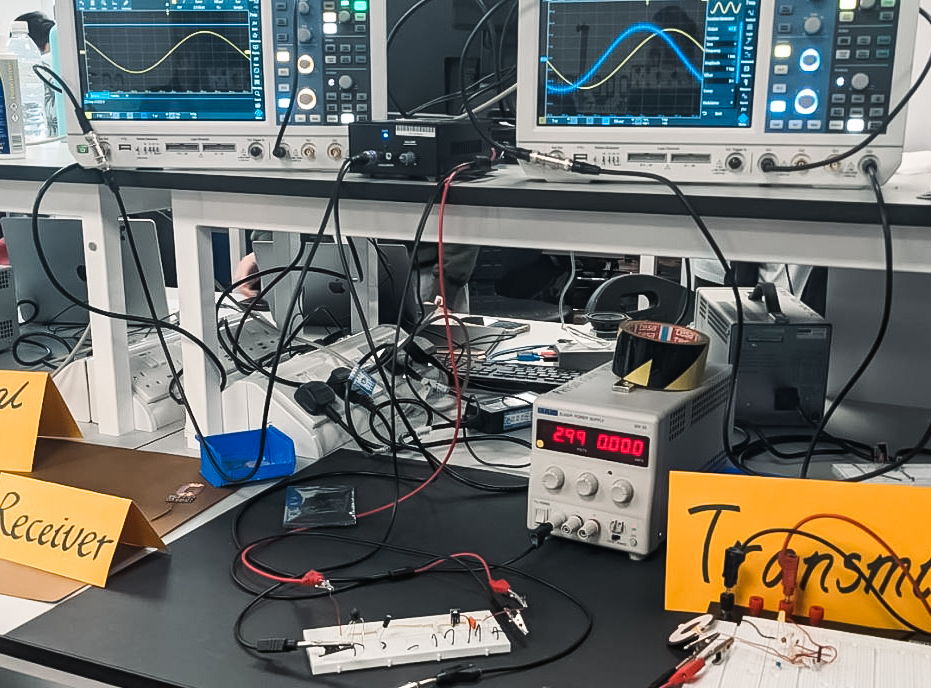
The second part of the day invited us to scatter like billiard balls to the countless different projects that the undergraduates had been working on. The intricacy of the work was truly magnificent: ultrasonic levitators, liquid-mirror telescopes, self balancing gyroscopes, the construction of lasers, voice changers, even detecting muons - one of the fundamental subatomic particles. With more than a hundred projects to see and interact with, this treasure chest of learning was entrancing. Physics seemed as big and majestic as Space itself. We didn't even feel hungry till we discovered we'd be getting a free lunch!
The day ended with a lecture on the Sun, and specifically, its sunspots which are temporary ‘spots’ which alter the amount of solar flares released by this ball of dust and gas. This has made scientists theorise that sunspots may be a natural cause of climate change. Alperton’s inspired young Physicists then split into smaller groups, some of whom stayed for a while longer at the university to learn from even more projects whilst others enjoyed a final stop in the surroundings of the university campus, including Hyde Park where the students were able to bond on their final day together before the holidays.
It is one thing learning about a subject from a textbook, but something completely different when you actually see it happen before your very eyes. A huge thanks to Mr Lazaroo who led and coordinated this trip alongside Mr Raj and Ms Khalil. This truly was an imperial march that Alperton Community School took to this outstanding university and it has inspired many students to study Physics in greater depth. As a friend of mine exclaimed after the first lecture, “This is what life is about!”
-Tariq Al-Azzawi, 11P-
Making a Volcano (Acid and Alkali Reactions)
This video experiment was used to demonstrate other substances that react in neutralisation reactions (reaction of an Acid and Base). In the demonstration Potassium Iodide and Hydrogen Peroxide were used.
The year 8 and 9 students made models of volcanos and had used vinegar and baking soda for their volcano eruptions (another neutralisation reaction). Here are some photos of the students making their volcanos:
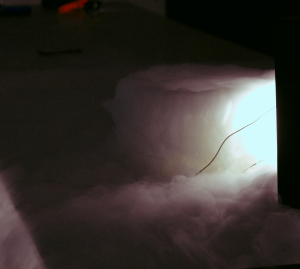 Dry ice, also known as solid carbon dioxide (CO2), undergoes sublimation, a unique phase transition in which a substance changes directly from a solid to a gas without passing through the liquid phase. The science behind this phenomenon is based on principles of temperature and pressure.
Dry ice, also known as solid carbon dioxide (CO2), undergoes sublimation, a unique phase transition in which a substance changes directly from a solid to a gas without passing through the liquid phase. The science behind this phenomenon is based on principles of temperature and pressure.
1. Temperature: Dry ice is incredibly cold, with a sublimation temperature of approximately -78.5 degrees Celsius (-109.3 degrees Fahrenheit) at standard atmospheric pressure (1 atmosphere or 101.3 kPa). This temperature is well below the freezing point of water. Because of this extreme cold, when dry ice is exposed to warmer surroundings, it begins to absorb heat energy.
2. Absorption of Heat: As the warmer air or surface around the dry ice transfers heat to it, the dry ice gains thermal energy. This energy causes the CO2 molecules within the solid dry ice to vibrate more rapidly, increasing their kinetic energy.
3. Breaking Molecular Bonds: The increased kinetic energy eventually overcomes the intermolecular forces holding the CO2 molecules together in the solid phase. These forces are relatively weak due to the small size of CO2 molecules and the absence of hydrogen bonding. As a result, the molecules break free from their fixed positions in the solid lattice.
4. Transition to Gas: Once a sufficient amount of thermal energy is absorbed, the CO2 molecules transform directly into the gaseous state. They move freely and rapidly in the gas phase, creating the characteristic fog or vapor cloud associated with dry ice sublimation.
5. Expansion: The rapid expansion of CO2 gas during sublimation can create a visible plume of "smoke" or vapor, which adds to the dramatic effect. In summary, dry ice sublimation occurs because of the transfer of heat energy from the surroundings to the cold dry ice, which causes the solid CO2 molecules to transition directly into a gas state, bypassing the liquid phase. This process is not only scientifically fascinating but also has practical applications in various fields, such as in creating theatrical effects, preserving perishable goods, and in laboratory experiments.
This video experiment illustrates an important physics law known as Boyles Law, which states that pressure is inversely proportional to volume at constant temperature.
As seen in the experiment, as the air is taken out of the glass jar containing the marshmallow, the pressure in the jar decreases, allowing the volume of the marshmallow to increase.
When the air is put back into the glass jar, the pressure increases and the volume of the marshmallow decreases.
Bread is made.
On Thursday 14 December, Dr Mahmoud kindly organised a cross-curricular enrichment activity involving a bread-making session with 25 Year 8 students, in line with the science curriculum for Year 8, where students have to learn about yeast and how it is used in food production.
Dr Mahmoud wanted to make the project practical and arranged an enrichment activity with the help of Ms Merghani, for students to get to use yeast to make their own bread. The project combined a minds-on approach, learning the theory in science, with the hands-on experience of seeing how yeast is used in bread making.
It was a lovely session and students got really engaged and made various bread shapes. At the end of the day they were allowed to take the bread home and share it with family.
Everyone participated, including our key groups, as part of the Science for All programme where every student can take part in science. Students were highly engaged and learned how science is linked to the everyday activities they come across.
Many thanks to Dr Mahmoud and Ms Merghani for organising this unique activity for our students.
Kindly see below some photos of the bread made by the students:
Over 13 weeks, our school students Mitesh, Faoziya, Bhavya and Tariq have mastered design and making skills, such as 3D printing, laser cutting, CAD, woodworking, electronics, and Arduino and brought their very own invention to life in the vibrant workshop of Imperial College London. Supported by Imperial experts and with access to world-class facilities, our students took part in the challenge to create prototypes of their inventions and presented their ideas to a panel of judges and family members on Saturday 9th December. Congratulations to all students who took part in this wonderful project.
Below you can see some photos with the students' work:
Utilising the idea of ‘classroom physics’ from the June 2023 edition of the Institute of Physics magazine, which focused on the topics of balance and stability, our Y10 triple science students engaged in a STEM activity (Balanced flight), where they learned how to make gliders using items such as paper, glue or cardboard. Once completed, students took their gliders out for a test run in the lovely sunny weather of Monday 20th of May.
Below are two images of some of our students' work. In the first image, no white-tac was used, and when launched into the air, the glider did not have a stable flight. In the second image, white-tac was added to the nose of the plane, which caused the centre of mass to move forward, and as a result, the plane had a more stable and balanced flight.
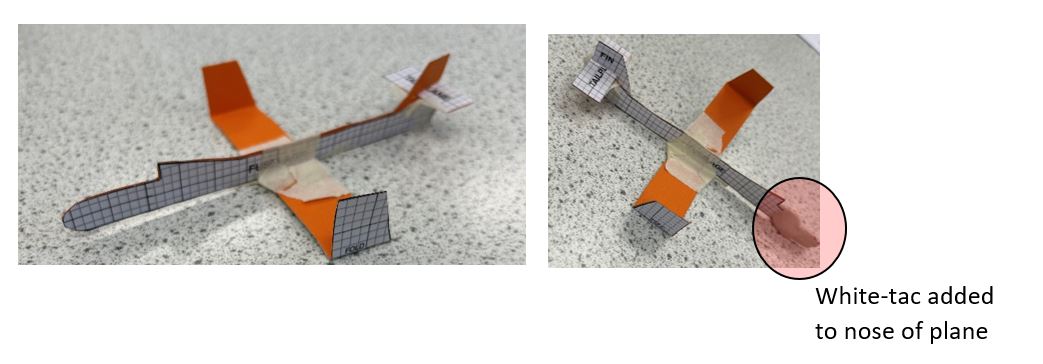
Engaging in this activity, students learned about real-world application of centre of mass and torque and why they are important in the design of planes.
At ACS we embed STEM into our timetabled lessons to make for a more engaging, effective and inclusive classroom. (Dr Mahmoud)